Early detection of infectious diseases is a cornerstone of effective veterinary healthcare. Leishmaniasis, caused by the parasite Leishmania infantum, remains a crucial challenge in canine medicine due to its potentially severe impact on dogs’ wellbeing.
What is Leishmania?
Leishmania is a genus of protozoan parasites transmitted primarily through the bites of sand flies. In canines, the species responsible for the condition known as canine leishmaniasis is Leishmania infantum. Once inside the host, these parasites can disseminate throughout various tissues, including the spleen, bone marrow, liver, and lymph nodes, sometimes taking months or even years to become symptomatic.
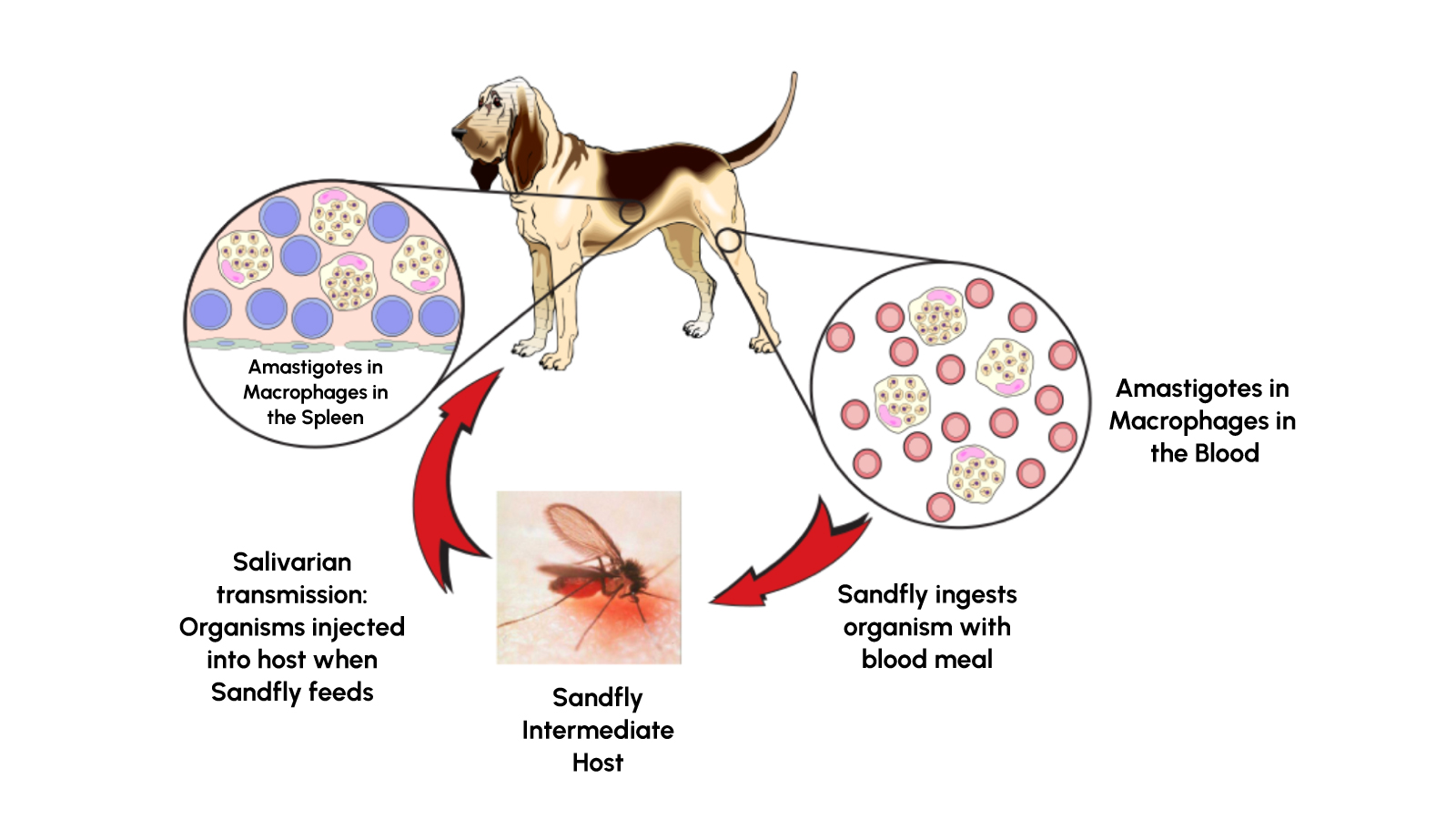
Signs and Symptoms in Canines
While some infected dogs remain asymptomatic, many develop pronounced clinical signs. Key symptoms include:
- Persistent fever and lethargy
- Loss of appetite and noticeable weight loss
- Vomiting, diarrhea, and potential nosebleeds
- Lesions on the skin and coat
- Weakness or lameness
The incubation period for canine leishmaniasis typically ranges between 3 to 18 months. However, because the disease can also present subclinically for extended durations, timely diagnosis is vital.
The Challenges of Diagnosis
Diagnosing canine leishmaniasis can be challenging, especially in the early stages of the infection. Traditional diagnostic approaches include tissue aspiration from the spleen, lymph nodes, or bone marrow. While definitive, these methods can be invasive and require specialized equipment and expertise.
Limitations of Delayed or Invasive Testing
- Prolonged Turnaround: More intricate diagnostic tests often require multiple steps, additional reagents, and skilled personnel.
- Invasiveness: Tissue aspiration procedures can cause discomfort and may risk complications.
- Risk of Transmission: Delayed diagnosis can prolong parasite transmission, raising concerns for dogs in endemic areas and for immunocompromised animals.
The Role of the Leishmania Rapid Antibody Test Kit
One effective solution to these diagnostic hurdles is the Leishmania Rapid Antibody Test Kit, designed to detect canine antibodies against Leishmania infantum. This rapid kit leverages immunochromatographic technology, providing quick and accurate resultsoften within 5 to 10 minuteswhile maintaining high sensitivity and specificity.

Key Features and Benefits for Healthcare Providers
- Speed and Efficiency: Rapid antibody test kits reduce the waiting period from days to minutes. Healthcare providers can make faster clinical decisions, optimizing patient care.
- High Sensitivity and Specificity: According to performance data, the test demonstrates a high sensitivity and specificity. These translate into fewer false results, boosting diagnostic confidence.
- User-friendly Procedure: The kit comes with all necessary components, including test cassettes and single use solution tubes. Blood collection is straightforward, and minimal training is needed to perform the test accurately.
- Minimal Sample Volume: Only 20 µL of serum or plasma is required, making sample collection less invasive and more convenient.
- Portability: The compact format and simple storage conditions (2°C-30°C) allow for robust and reliable performance even in resource limited settings.
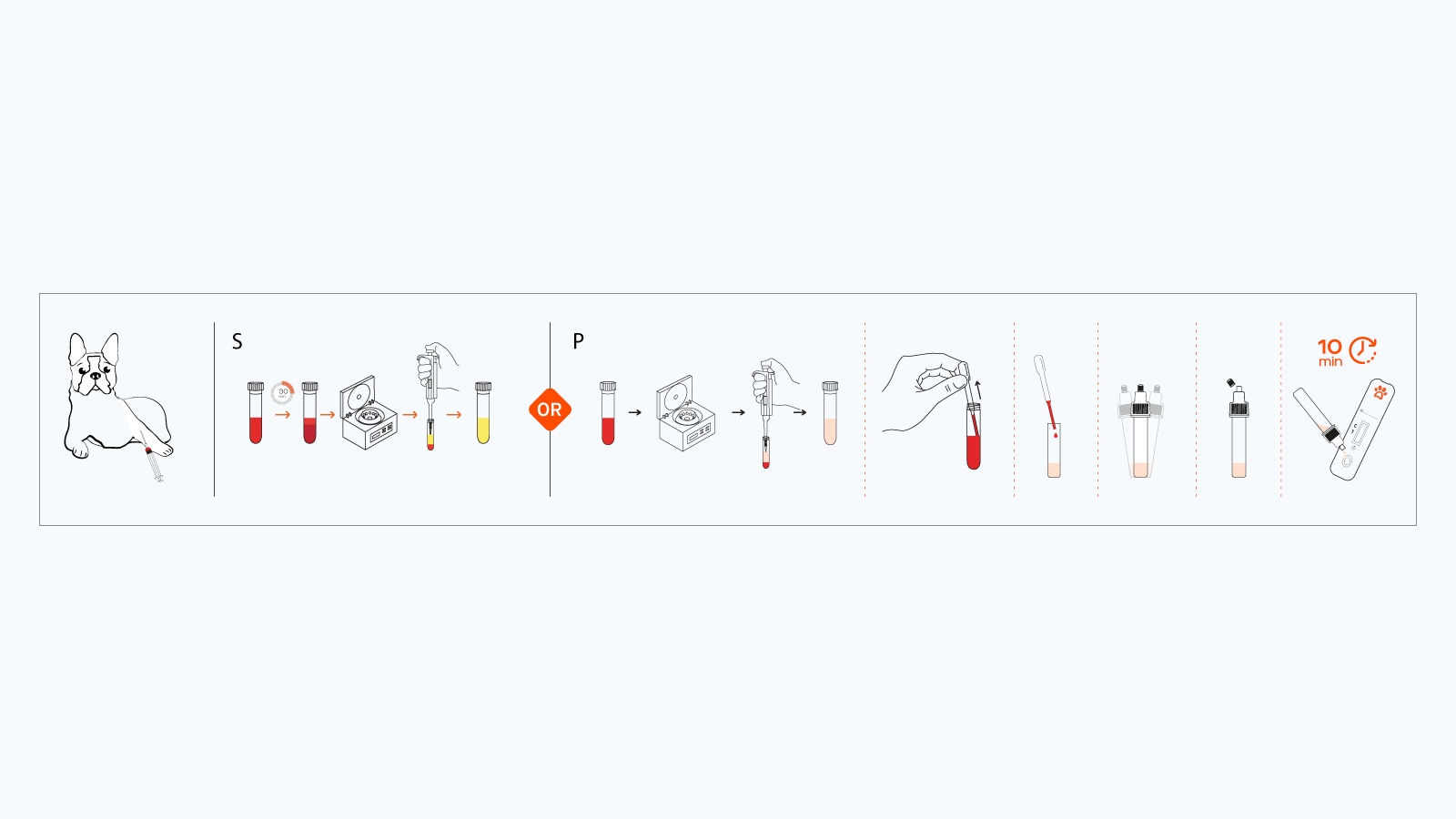
How the Test Works
The Leishmania Rapid Antibody Test Kit uses lateral flow and immunochromatographic principles. When a serum or plasma sample containing Leishmania antibodies is mixed with the provided solution, the complex travels across a nitrocellulose membrane where a high quality recombinant Leishmania antigen (rK39) is immobilized.
- Sample Preparation
- Draw canine blood into a non-anticoagulant tube (for serum) or an anticoagulant tube (for plasma).
- Allow the blood to clot (if collecting serum) and centrifuge to obtain the serum or plasma.
- Mix the Solution
- Combine 20 µL of the sample with the solution in the kit’s single use bottle.
- Gently mix by sealing the cap and inverting if needed.
- Apply the Sample
- Dispense three drops of the sample-solution mixture onto the sample well of the cassette.
- Interpretation of Results (Read within 5-10 minutes)
- Positive: Two red bands (T-line & C-line) appear.
- Negative: Only the C-line is visible.
- Invalid: C-line does not appear.
Best Practices and Safety Measures
- Professional Use Only
This video lateral flow test kit is intended strictly for veterinary professionals. Proper adherence to biosafety precautions is a must.
- Storage Conditions
Avoid exposing the test to direct sunlight. Keep the humidity.
- Handling Requirements
All test components and patient samples must be treated as potentially infectious. Thorough decontamination and proper disposal practices should follow local medical waste regulations.
Integrating Rapid Testing into Veterinary Practice
When combined with clinical judgment and other laboratory findings, rapid testing significantly improves patient outcomes. By quickly flagging positive cases, healthcare providers can initiate further confirmatory tests or begin management protocols (which may include antiparasitic therapy and supportive care).
Advantages for Vets and Clinics
- Reduced Diagnostic Uncertainty: Timely detection helps rule out or confirm leishmaniasis, guiding more targeted interventions.
- Enhanced Client Confidence: Pet owners appreciate quick, reliable, and minimally invasive diagnostic solutions, boosting clinic credibility.
- Streamlined Workflow: Minimal waiting periods reduce bottlenecks in highthroughput environments, optimizing clinic efficiency.
For healthcare providers, efficient and accurate testing for canine leishmaniasis is essential. The Leishmania Rapid Antibody Test Kit offers a convenient and highly reliable diagnostic tool that aligns with the clinical and operational demands of modern veterinary practice. By adopting rapid testing, you can enhance early detection, speed up patient care, and mitigate the spread of this potentially severe disease.
References
- World Health Organization (WHO). [Leishmaniasis](https://www.who.int/newsroom/factsheets/detail/leishmaniasis)
- Ready, P.D. “Epidemiology of visceral leishmaniasis,” Clinical Epidemiology, vol. 6, no. 56, 2014, pp. 423437.
